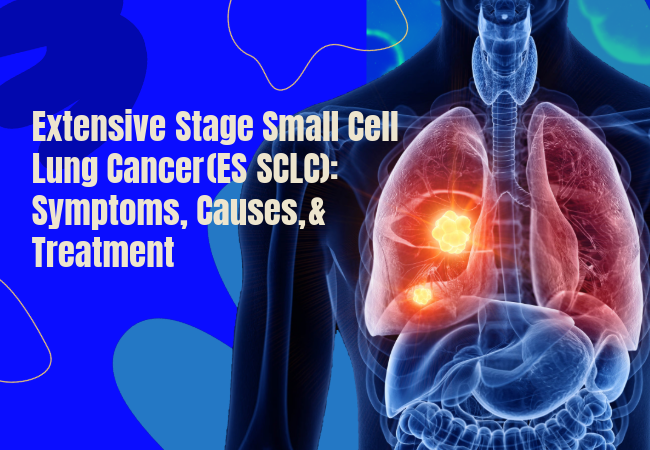
Small-cell lung cancer (SCLC) makes up 10 to 15% of lung cancer cases. SCLC is known for its rapid progression and widespread metastasis to various organs and lymph nodes. Roughly 70 to 80% of patients with SCLC have Extensive‑stage small cell lung cancer (ES-SCLC) at the time of initial diagnosis.
Extensive stage small cell lung cancer (ES-SCLC) is a very advanced type of cancer that is not just limited to one side of the chest and the nearby lymph nodes. It can also spread to more distant parts of the body, like the liver, bones, adrenal glands, or even the brain.
Early recognition of red‑flag symptoms, appreciation of modifiable risk factors, and timely therapy—including newly authorised agents such as Hetronifly (serplulimab) and Cosela (trilaciclib)—are important to improving outcomes.
Key Symptoms of ES‑SCLC:
Because SCLC proliferates quickly, symptoms may occur all of a sudden and progress within weeks:
- Persistent cough
- Chest pain
- Hoarseness
- Dyspnoea and wheezing
- due to central airway
- obstruction
- Unexplained weight loss
- Anorexia
- Profound fatigue
- Paraneoplastic syndromes (e.g., SIADH causing hyponatraemia, Cushing’s syndrome)
- Neurologic deficits occur when metastases involve the brain or spinal cord
Many patients are diagnosed with advanced-stage SCLC cancer due to the symptom overlap with COPD and other smoke-associated lung diseases.
Causes & Molecular Pathogenesis:
Tobacco use continues to be correlated with over 95% of small cell lung cancer (SCLC) cases. SCLC pathogenesis is characterized by activation of processes such as carcinogen-induced TP53 and RB1 loss and MYC amplification, and DLL3 over-expression. Extracted carcinogens, as well as occupational SCLC associated with asbestos and radon, as well as secondhand smoke inhalation, raise the cumulative risk. Never-smoker ES-SCLC, however, is very, very rare.
Established Risk Factors:
- Cigarette Smoking – The number of pack years directly connects to the rate of cigarette smoking-related death and disease.
- Age & Sex – Peak diagnosis occurs at 60 to 70 years; incidence historically greater in men but rising in women because of smoking trends.
- Comorbid Lung Disease – COPD and Emphysema share some of the same processes; chronic inflammation creates and sustains some inflammation, which helps solid tumors to grow.
- Occupational/Environmental Exposures – The occupation of uranium mining, and radon exposure in unstylish living quarters, and diesel particulate emissions build toward the risk in the area.
- Family History – There is some increase in the prevalence of SCLC in the first-degree relatives of lung cancer patients, but it is modest.
Diagnostic Work‑up & Staging:
When a doctor first suspects small cell lung cancer (SCLC), they perform specific imaging tests and biopsies to assess the cancer’s progression and formulate a treatment strategy:
- Contrast Chest CT Scan – A specialized chest X-ray assesses the lung tumor’s dominant size and any associated lymphadenopathy.
- Brain MRI – Since small cell lung cancer has a penchant for metastasizing to the brain early on, an MRI is done to find any concealed lesions pre-symptomatically.
- Whole‑Body PET‑CT – It locates the hotspots and metastasis of the disease for the liver, bone, or even the adrenal glands, which would potentially be overlooked by a standard CT scan.
- Bronchoscopy with Biopsy – This procedure involves the passing of a thin camera tube to the airways for the collection of a tissue biopsy which is examined microscopically for confirmation of the diagnosis.
In case any of these tests indicate that the cancer has spread outside one lung and its nearby lymph nodes—for example, to the opposite liver, lung, bones, or brain—the disease is classified as extensive‑stage small cell lung cancer (ES‑SCLC).
Treatment & Management:
For years, extensive-stage small cell lung cancer (ES-SCLC) has been treated with platinum-etoposide chemotherapy. Patients often don’t experience long-lasting remission, and out-of-control bone marrow suppression is common. Two new medications are now changing this. Hetronifly (serplulimab) is an anti-PD-1 antibody given alongside chemotherapy as a first-line treatment and improves overall survival. COSELA™ (trilaciclib) is the first FDA-approved myeloprotective drug to preserve bone-marrow function without losing control of the cancer. These agents maximise anti‑cancer efficacy while minimising haematologic toxicity for patients with ES‑SCLC.
Hetronifly (Serplulimab):
Serplulimab is an anti‑PD‑1 monoclonal antibody. It is recently authorised in multiple regions—including the European Union (EU)—for first‑line treatment of adult ES‑SCLC along with platinum/etoposide. In a pivotal Phase III trial (ASTRUM‑005), serplulimab plus chemotherapy improved median overall survival to 15.4 months versus 10.9 months with chemotherapy alone. This represents the longest overall survival (OS) reported in this setting.
Cosela (Trilaciclib):
Trilaciclib is a cyclin‑dependent kinase 4/6 inhibitor. It is approved by the U.S. FDA to decrease the incidence of chemotherapy‑induced myelosuppression in adults receiving platinum/etoposide‑ or topotecan‑based regimens for ES‑SCLC. It is administered by IV infusion 30 minutes before chemotherapy. Trilaciclib is not an anti‑cancer agent. It optimises chemotherapy tolerability without compromising tumour response.
Emerging Therapies & Clinical Trials:
- Antibody–drug conjugates (ADCs): Tarlatamab (DLL3‑targeted) shows durable activity in relapsed SCLC.
- PARP Inhibitors: Talazoparib plus temozolomide under investigation for biomarker‑selected populations.
- Bispecific T‑cell Engagers: Trials are in progress to improve immune redirection strategies.
Novel therapies and treatment options should be accessed and contributed to by patients through trials whenever possible to improve standards of care for the future.
Outlook & Conclusion:
Although treating extensive-stage small cell lung cancer ( ES-SCLC) is still quite challenging, new treatments are emerging. Cosela protects bone marrow, enabling cancer patients to complete chemotherapy with less toxic aftermath, and Hetronifly, as an immune checkpoint blocker, helps the body to better fight against cancer.
These medicines are not yet approved or available in the Indian market, but Indian Pharma Network (IPN) can help doctors and hospitals get them legally through the provision called the Named Patient Program. IPN partners with EU‑GMP and FDA‑certified suppliers to deliver genuine medicinal products quickly and safely, giving patients access to the latest treatments
Disclaimer: This article is for educational purposes only. All the decisions related to the treatment must align with local regulatory approvals and individual patient factors. Always consult oncology specialists and official prescribing information.
Is Hetronifly approved for commercial use in India?
No. As of today, Hetronifly is not CDSCO‑approved. Access is limited to legal Named‑Patient Import or compassionate‑use programmes, each requiring a physician’s prescription and CDSCO authorisation before shipment.
Who is a reliable Cosela importer in India?
Indian Pharma Network (IPN) is a trusted Cosela (trilaciclib) importer in India. Although this therapeutic drug is not commercially approved in India, IPN can facilitate it under the Named‑Patient Program (NPP).
How can I buy Hetronifly and Cosela in India?
Although Hetronifly and Cosela are not yet approved in India. But, Indian patients may legally buy Hetronifly and Cosela in India via the regulatory‑sanctioned Named Patient Program, facilitated by trusted import partners like Indian Pharma Network (IPN).