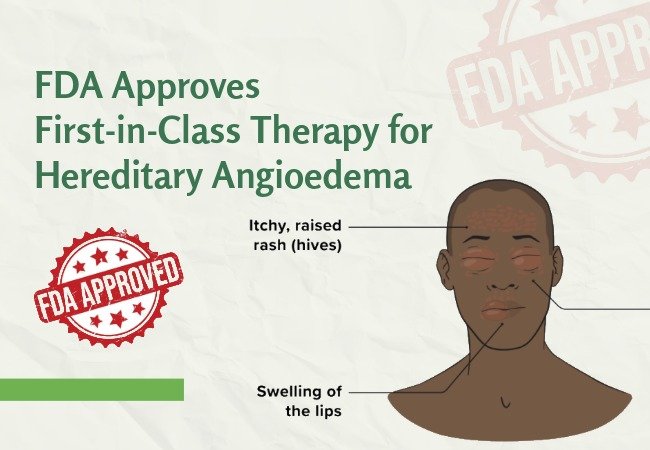
Dawnzera (Donidalorsen) is now approved by the U.S. Food and Drug Administration (FDA) as the first-ever and only therapy targeting RNA for the prevention of attacks of hereditary angioedema (HAE) for patients 12 years and older. This is the first move by the USA to provide treatment to a patient suffering from an extremely rare genetic condition, which can lead to swelling that is unpredictable and, at times, can even lead to death.
What is Hereditary Angioedema (HAE):
Hereditary angioedema (HAE) is a rare disease caused by genetic mutations that cause low levels or dysfunction of C1 inhibitor, a protein involved in controlling inflammation. The HAE patients experience sudden swelling in areas such as the gastrointestinal tract, face, limbs, and airway. When swelling affects the throat, it can become a medical emergency. The burden of the disease often extends beyond physical symptoms, as patients live with uncertainty about when attacks may occur.
About the FDA Approval:
Dawnzera is an antisense oligonucleotide therapy. It is designed to decrease the production of prekallikrein (PKK), a protein associated with the swelling cascade in HAE. By targeting this pathway, the therapy stops the attacks before they happen. According to the FDA label, Dawnzera can be administered through a subcutaneous (SC) injection every 4 or 8 weeks. The new approval offers patients and physicians flexibility in preventive care.
Evidence from Clinical Studies:
The FDA approval is supported by evidence from phase 3 clinical trials. Results demonstrated that Dawnzera reduced the frequency of HAE attacks by around 81% compared to placebo during a 24-week treatment period. Trial participants also reported fewer serious attacks and reduced need for on-demand medicine. The most common side effects included headaches, injection site reactions, and mild fatigue.
What This Means for Patients:
The approval of Dawnzera adds a new preventive option to the limited treatments available for hereditary angioedema (HAE). It provides healthcare providers (HCPs) with another tool to help patients manage this condition properly under regulatory guidance. For individuals and families living with the condition HAE, this development represents progress toward more consistent and predictable disease management.
Reference:
Ionis.com