Home » News
FDA Approves First Intranasal Diuretic, Enbumyst
Enbumyst (bumetanide nasal spray), developed by Corstasis Therapeutics, has received approval from the U.S. Food and Drug Administration (FDA). It ...
Moderna’s 2025–2026 COVID-19 Vaccines Receive FDA Approval
The 2025–2026 formulations of Moderna's COVID-19 vaccines, Spikevax® and mNexspike®, have received approval from the U.S. Food and Drug Administration ...
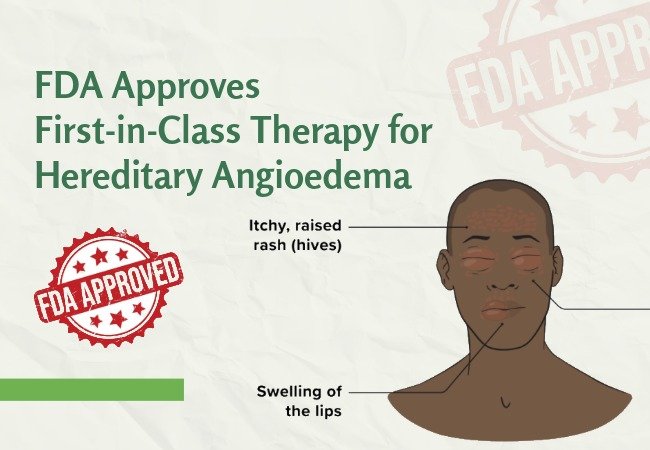
FDA Approves First-in-Class Therapy for Hereditary Angioedema
Dawnzera (Donidalorsen) is now approved by the U.S. Food and Drug Administration (FDA) as the first-ever and only therapy targeting ...
We’re Heading to ESMO Congress 2025 in Berlin
We’re pleased to confirm our participation at the ESMO Congress 2025 in Berlin, Germany, one of the most important oncology ...
Takeda’s Qdenga Vaccine: A Breakthrough in Dengue Prevention Coming to India
Takeda Pharmaceuticals recently declared its plans to launch Qdenga, its dengue vaccine, in India by next year. This vaccine could ...
Indian Company Can Manufacture Risdiplam for Spinal Muscular Atrophy (SMA)
In a major development, a leading Indian pharmaceutical company, Natco Pharma, has been given approval to produce Risdiplam. This is ...
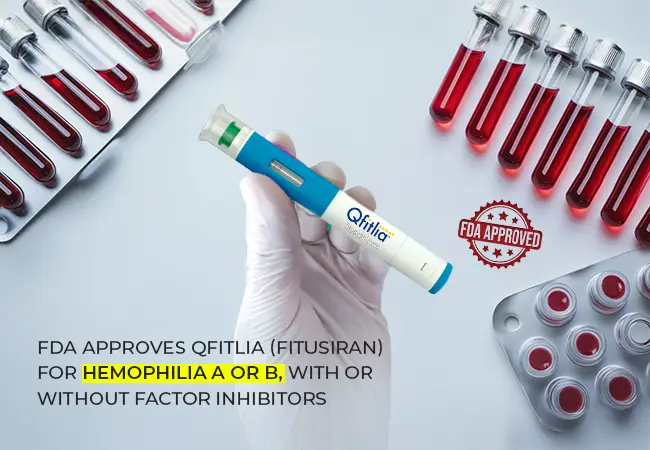
FDA Approves Qfitlia (Fitusiran) for Hemophilia A or B, with or without Factor Inhibitors
Introduction: The Promise of Qfitlia for Hemophilia TreatmentThe FDA has authorized Qfitlia (Fitusiran), a new treatment for both Hemophilia A ...
FDA Approves First Treatment for a Rare Lipid Storage Disease
On February 21, 2025, the Food and Drug Administration of the United States (FDA) approved Ctexli (chenodiol) for CTX therapy ...
FDA Approves Tenecteplase for Acute Ischemic Stroke (AIS)
Recently, the U.S. Food and Drug Administration (FDA) approved TNKase (tenecteplase) for the use of treating adults with acute ischemic ...