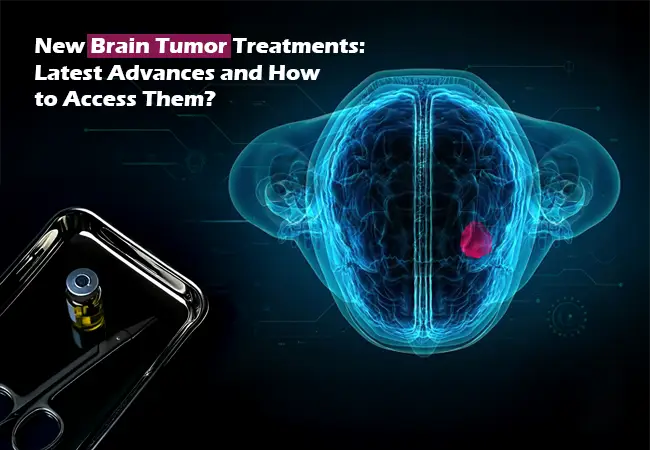
In both adults and children, brain tumors are considered one of the most complicated and life-altering conditions in oncology. The tumors can be classified as primary (originating in the brain) or secondary (spread from other parts of the body). The treatment for them is effective but complicated owing to the delicate region of the tumor, possible neurological complications, and multiple types of tumors present.
The most common types of brain tumors include:
- Glioblastoma
- Multiforme (GBM)
- Astrocytoma
- Oligodendroglioma
- Medulloblastoma
- Ependymoma
- Meningioma
- Brain Metastases (from other cancers)
The past few years have seen the emergence of new therapies such as targeted therapies, immunotherapies, tumor-treating fields (TTF), and gene-directed therapies, which are significantly changing the treatment experience for patients diagnosed with high-grade or recurrent brain tumors.
Standard Treatments for Brain Tumors:
The first step in addressing an issue usually involves conducting an MRI or CT scan, procuring a biopsy, and conducting molecular analysis to ascertain the type and grade of the tumor. Treatment possibilities include:
- Surgery – Either partial or complete removal of the tumor.
- Radiation Therapy – Cancerous cells that remain after surgery are to be destroyed using radiation.
- Chemotherapy – Treatment via drugs either locally or throughout the body to stop the tumor from growing, can be done either systematically or locally.
- Targeted Therapy – Uses medication that prevents more specific changes in genes or proteins in the cells in the tumor.
- Immunotherapy – Treatment that helps the immune system of the patient stimulate and fight against cancer.
- Tumor Treating Fields (TTF) – Uses low-intensity electric fields to interfere with the reproduction of cancer cells.
Newly Approved Medicines and Treatments for Brain Tumors:
The following are the most cutting-edge treatments recently granted, which enhance the hope granted to people suffering from brain tumors, particularly those with aggressive ones or those most difficult to treat.
Temodar (temozolomide):
- Type: Alkylating Chemotherapy
Indication: Glioblastoma Multiforme (GBM), Anaplastic Astrocytoma - Mechanism: Crosses the blood–brain barrier to damage DNA in rapidly dividing tumor cells
- Approval: FDA-approved as first-line therapy for newly diagnosed GBM
Lomustine (CCNU):
- Type: Oral Nitrosourea Chemotherapy
- Indication: Recurrent high-grade gliomas
- Mechanism: Disrupts DNA replication and transcription in cancer cells
- Approval: Widely approved for use in relapsed gliomas; used off-label in combinations
Avastin (bevacizumab):
- Type: Monoclonal Antibody (Anti-VEGF)
Indication: Recurrent Glioblastoma - Mechanism: Inhibits vascular endothelial growth factor (VEGF) to cut off the tumor’s blood supply
- Approval: FDA-approved for recurrent GBM
Optune (Tumor Treating Fields):
- Type: Medical Device (Wearable Therapy)
- Indication: Newly diagnosed and recurrent Glioblastoma
- Mechanism: Delivers low-intensity alternating electric fields to disrupt cancer cell division
- Approval: FDA-approved in combination with temozolomide
Rozlytrek (entrectinib):
- Type: Tyrosine Kinase Inhibitor (TKI)
- Indication: NTRK gene fusion-positive solid tumors, including brain tumors
- Mechanism: Inhibits TRK fusion proteins involved in cancer growth
- Approval: FDA-approved for NTRK fusion-positive cancers with CNS involvement
Vitrakvi (larotrectinib):
- Type: Selective TRK Inhibitor
- Indication: NTRK fusion-positive CNS and solid tumors in adults and children
- Mechanism: Blocks TRK fusion proteins to halt tumor growth
- Approval: FDA-approved for NTRK fusion-positive tumors regardless of location
Tibsovo (ivosidenib):
- Type: IDH1 Inhibitor
Indication: IDH1-mutated gliomas - Mechanism: Inhibits the mutant IDH1 enzyme to reduce cancer cell proliferation
- Approval: FDA-approved for hematologic cancers; expanded trials for IDH1-mutant glioma
Onivyde (irinotecan liposome):
- Type: Liposomal Chemotherapy
- Indication: Under investigation for recurrent malignant gliomas
- Mechanism: Enhances delivery and persistence of irinotecan in tumor tissue
- Approval: FDA-approved for pancreatic cancer; studied in glioma treatment combinations
Zolbetuximab (claudin 18.2 antibody):
- Type: Investigational Monoclonal Antibody
- Indication: Claudin 18.2-expressing glioblastoma
- Mechanism: Binds claudin 18.2 to trigger immune cell-mediated tumor cell death
- Approval: Under clinical investigation; not yet FDA-approved for CNS tumors
Afinitor (everolimus):
- Type: mTOR Inhibitor
- Indication: Subependymal Giant Cell Astrocytoma (SEGA) in Tuberous Sclerosis Complex (TSC)
- Mechanism: Blocks mTOR pathway to slow tumor growth and cell proliferation
- Approval: FDA-approved for SEGA associated with TSC
Verzenio (abemaciclib):
- Type: CDK4/6 Inhibitor
Indication: Under clinical trial for recurrent glioblastoma and pediatric brain tumors - Mechanism: Inhibits cyclin-dependent kinases to reduce cancer cell replication
- Approval: FDA-approved in breast cancer; under investigation for brain tumors
Opdivo (nivolumab):
- Type: PD-1 Immune Checkpoint Inhibitor
Indication: Recurrent GBM, CNS metastases from melanoma, and lung cancer - Mechanism: Reactivates immune cells to recognize and destroy tumor cells
- Approval: Approved for multiple cancers; clinical trials ongoing in gliomas
Retevmo (selpercatinib):
- Type: RET Inhibitor
Indication: RET fusion-positive tumors, including CNS metastases - Mechanism: Selectively blocks RET fusion proteins driving tumor growth
- Approval: FDA-approved for RET-altered thyroid, lung, and other tumors with brain involvement
Dabrafenib + Trametinib:
- Type: BRAF + MEK Inhibitor Combination
- Indication: BRAF V600E-mutant gliomas (adult and pediatric)
- Mechanism: Blocks BRAF and MEK pathways to prevent tumor cell signaling
- Approval: FDA-approved for BRAF V600E-positive solid tumors, including CNS
Stivarga (regorafenib):
- Type: Multi-Kinase Inhibitor
- Indication: Recurrent Glioblastoma (in clinical trials)
- Mechanism: Inhibits multiple pathways involved in tumor growth and angiogenesis
- Approval: Approved for colorectal and liver cancer; clinical use in glioma under study
Accessing New Brain Tumor Treatments in India and Other Countries:
Unfortunately, many of the above-listed medicines are not yet approved or widely available in various countries. Through Named Patient Programs (NPPs) and regulatory-compliant import pathways, patients and doctors can legally access these critical treatments.
Indian Pharma Network (IPN) is India’s Leading Partner in Named Patient Access and Import of Rare & Hard-to-Find Medicines.
We facilitate the supply of advanced brain tumor therapies like Optune, Avastin, Rozlytrek, Tibsovo, and others based on a valid prescription and regulatory permission.
If you or a loved one has been diagnosed with a brain tumor and urgently needs access to a newly approved or investigational treatment not available in your country, Indian Pharma Network (IPN) is ready to support you. Contact us to explore your brain tumor treatment options today.
Disclaimer: This content is for informational purposes only and does not replace medical advice. Always consult your treating physician before starting any new medicine or therapy.
How can I access new brain tumor treatments in India or other countries?
Indian Pharma Network (IPN) legally helps import medicines through Named Patient Programs which are not available in your country.
Can I buy Optune or Avastin for brain tumors in India?
Yes, with a valid prescription, we can assist in sourcing Optune, Avastin, and other approved or investigational brain tumor therapies from certified global suppliers.
What is the price of Temozolomide or Rozlytrek in India?
Prices of these therapeutic drug may vary depending on the manufacturer, dosage, and country of origin. Contact us today for the most accurate and updated quote.
Are these drugs available globally through IPN?
Yes. Indian Pharma Network (IPN) supplies to more than 150 countries through legal channels and supports patients across the world in accessing the newest cancer medicines.
Do you offer cold-chain shipment for brain tumor therapies?
Yes, we ensure cold-chain validated delivery for all temperature-sensitive medicinal products to maintain their potency and safety during transport.
Why Choose Indian Pharma Network (IPN)?
- Biggest Indian Company in the
- Named Patient Supply Segment
- 30+ Years of Global Pharma Access Experience
- 10,000+ Patients Served Across 150+ Countries
- 15,00,000+ Product Lines Facilitated
20+ Therapeutic Areas Supported, including oncology and neurology - Cold Chain Handling of Specialty Medications
- Access to US FDA, EMA, UK MHRA & WHO-GMP Certified Drugs
CDSCO & DGFT Regulatory Compliance for Import into India