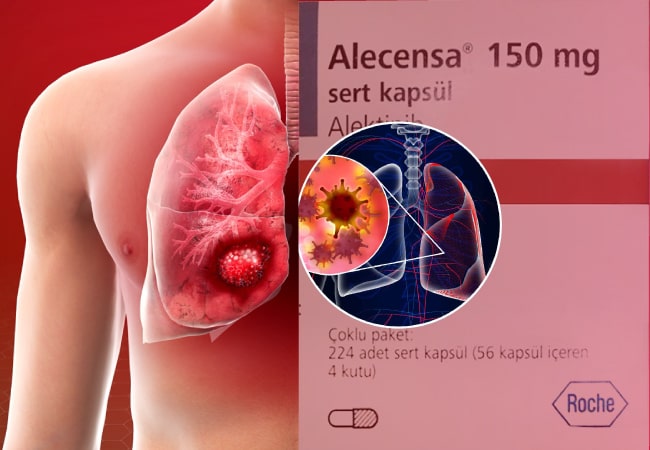
In a groundbreaking move, the Food and Drug Administration (FDA) has given its seal of approval to Alectinib (marketed as Alecensa) as adjuvant therapy for patients grappling with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) following tumor resection. This marks a significant stride as it becomes the first-ever ALK inhibitor tailored specifically for patients with surgically removed early-stage NSCLC tumors.
The approval, heralded by a press release from Genentech, the drug’s manufacturer, stems from compelling data culled from the phase 3 ALINA study (NCT03456076). Results from this study showcased a remarkable 76% reduction in the risk of disease recurrence or death among patients treated with alectinib compared to those subjected to platinum-based chemotherapy. Notably, participants in the trial had undergone complete resection of IB (tumors of at least 4 cm) to IIIA ALK-positive NSCLC.
Moreover, an exploratory analysis within the study hinted at enhanced central nervous system-disease-free survival rates among patients administered with alectinib, underlining its potential in mitigating disease progression in critical areas.
Dr. Levi Garraway, Genentech’s Chief Medical Officer and head of Global Product Development, hailed the approval as a monumental leap forward in cancer care. He emphasized the unprecedented efficacy of Alecensa, which significantly outperformed conventional chemotherapy, offering new hope to those battling early-stage ALK-positive lung cancer.
The safety profile of alectinib, as observed in the ALINA study, remained consistent with previous trials, with no alarming safety red flags. Common adverse reactions included constipation, hepatotoxicity, myalgia, rash, fatigue, COVID-19, and cough, as outlined in the FDA’s release.
For administration, the FDA recommends a 600-mg oral dose of alectinib to be taken twice daily with food over a span of two years or until the onset of unacceptable toxicity or disease recurrence.
Ken Culver, Director of Research and Clinical Affairs at ALK Positive, Inc., hailed the approval as a pivotal moment for patients grappling with early-stage ALK-positive lung cancer. He underscored the importance of widespread testing for ALK and other biomarkers to ensure patients receive the most fitting treatment regimen.
In essence, the FDA’s green light for alectinib ushers in a new era of personalized treatment options, offering renewed optimism to patients and clinicians alike in the relentless battle against NSCLC. As medical science continues to advance, such milestones reaffirm the power of innovation in reshaping the landscape of cancer care.
References:
https://www.oncnursingnews.com/view/fda-approves-first-alk-inhibitor-for-adjuvant-therapy-in-early-stage-nsclc
https://www.gene.com/media/press-releases/15023/2024-04-18/fda-approves-genentechs-alecensa-as-firs
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alectinib-adjuvant-treatment-alk-positive-non-small-cell-lung-cancer