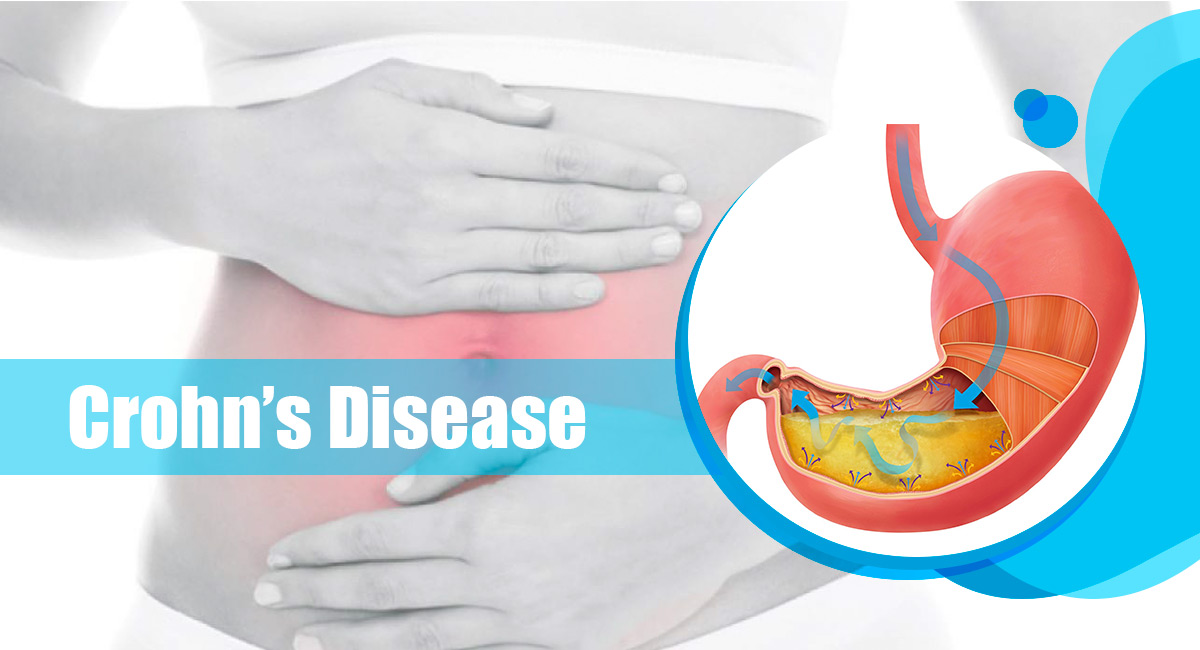
Ustekinumab is also known by the brand name Stelara. It is used to treat moderate to severe active Crohn’s Disease and moderate to severe active Ulcerative Colitis in adults. It can be an option when certain other therapies including steroids, immunosuppressants (such as azathioprine, mercaptopurine, methotrexate) or biologic drugs such as anti-TNF’s infliximab, adalimumab or golimumab haven’t been effective. Or if other therapies have stopped working or you’ve experienced adverse events that are hard to manage.
Mechanism: The ustekinumab injection falls under the group of medications named ‘biological drugs’. These drugs are produced by a biological, rather than chemical, process. Ustekinumab is a synthetic (man-made) antibody that is created inside the living cells. It is mainly an anti-Interleukin biological drug. It targets a couple of proteins in the body named interleukin-12 (IL-12) and interleukin-23 (IL-23). IL-12 and IL-23 are the naturally produced in the body in order to help fight the infections by temporarily causing inflammation. IL-12 and IL-23 are increased in IBD and contribute to the ongoing, or chronic, inflammation in the digestive system. This medication binds to both IL-12 and IL-23 which prevents them from acting, helping to relieve inflammation and symptoms. It is known as an ‘immunosuppressant’ because it dampens down the mechanism of the immune system.
Duration of Treatment: Individuals responds differently when taking any new medication, and ustekinumab doesn’t work for everyone. You may feel good as early as 3 weeks after taking ustekinumab, but most individuals who respond to ustekinumab start feeling better within 6 weeks. In some cases it may take a bit take longer. In case you respond to treatment with ustekinumab and have no severe side effects, you may be put onto a planned course of therapy lasting up to a year, following which it can be extended. Your treatment plan will need to be reassessed at least every one year to see whether ongoing ustekinumab therapy is appropriate for you. In case you are in stable remission, it can be decided that you can stop taking ustekinumab dose. But If after stopping the treatment you become unwell again, you should have the option to restart. You may be taken off this.edication if you have any severe side effects or in case you have not responded well enough within 16 weeks of initiating your treatment.
Dosage: Ustekinumab comes in the pre-filled syringe containing 90 mg. It can’t be taken by mouth (in tablet form) because the digestive system would destroy it. The initial dose of ustekinumab is approximately 6 mg for every kg you weigh administeted as a drip into a vein in the arm, an intravenous (IV) infusion. All the following doses are 90 mg regardless of what your weight is, and these are all administered as an injection under the skin (subcutaneously).
Administration: The first dose of ustekinumab is administeted in hospital as a drip into a vein in your arm – an intravenous (IV) infusion. The next dose is administeted as an injection under the skin – a subcutaneous injection 8 weeks later. You will then have the further injections every 8 or 12 weeks. Your health specialist will decide whether you will need to take ustekinumab every 8 weeks or every 12 weeks. To begin with, a health specialist will give you the injections. Once you are used to having the treatment, they will teach you the technique so that you can easily self-inject. If you prefer, a family member or friend can be trained to administer the injections.
Effect of Ustekinumab: Ustekinumab can be effective in order to improve symptoms and in bringing about and maintaining the remission in individuals with moderate to severe Crohn’s Disease and moderate to severely active UC. It means that the inflammation in the gut is effectively decreased and your symptoms go away or significantly improve. Taking the drug ustekinumab may also mean you no longer have to take steroids. Certain studies suggest ustekinumab may also be useful in treating perianal fistulating Crohn’s Disease. A perianal fistula connects the anal canal (back passage) to the surface of the skin just near the anus.
Checks Prior to Taking Ustekinumab: Before you start Ustekinumab treatment, it is crucial to make sure that it is right for you. Make sure the team treating you know:
- in case you have ever had an allergic reaction to ustekinumab or latex, or in case you are allergic to any of its other ingredients.
- in case you have any history of TB (tuberculosis) or any exposure to individuals with TB. You need not be given ustekinumab in case you have active TB. In case you have underlying, inactive TB, this must be treated prior to starting ustekinumab.
- in case you have a history of infections, have an ongoing infection or have symptoms such as feeling feverish or generally unwell. In case you’ve an infection your therapy may need to be postponed.
- in case you are having or have ever had injections in order to treat the allergies. It is unknown if ustekinumab affects these.
- in case you are pregnant, planning to get pregnant, or are breastfeeding.
- in case you are taking or have recently taken any other drugs, including other biological drugs such as adalimumab or infliximab or immunosuppressive medicines such as mercaptopurine, methotrexate or azathioprine.
- in case you have recently had or will have any vaccinations.
- difficulty breathing or swallowing
- swelling in any part of the body
- dizziness or light-headedness
- redness of the skin
- an itchy rash or itchy skin.
- pain in the part of your arm where the infusion needle was inserted
- chills, shivering, or high fever
- fever, flu-like symptoms
- night sweats
- feeling tired
- short of breath
- a cough that won’t go away
- superficial fungal infections
- warm, red, painful skin, or a painful skin rash with blisters
- burning when passing urine
- sore throat
- common cold
- dizziness
- headaches
- diarrhoea
- nausea or vomiting
- itching
- back pain, muscle pain, joint pain
- fatigue or feeling tired