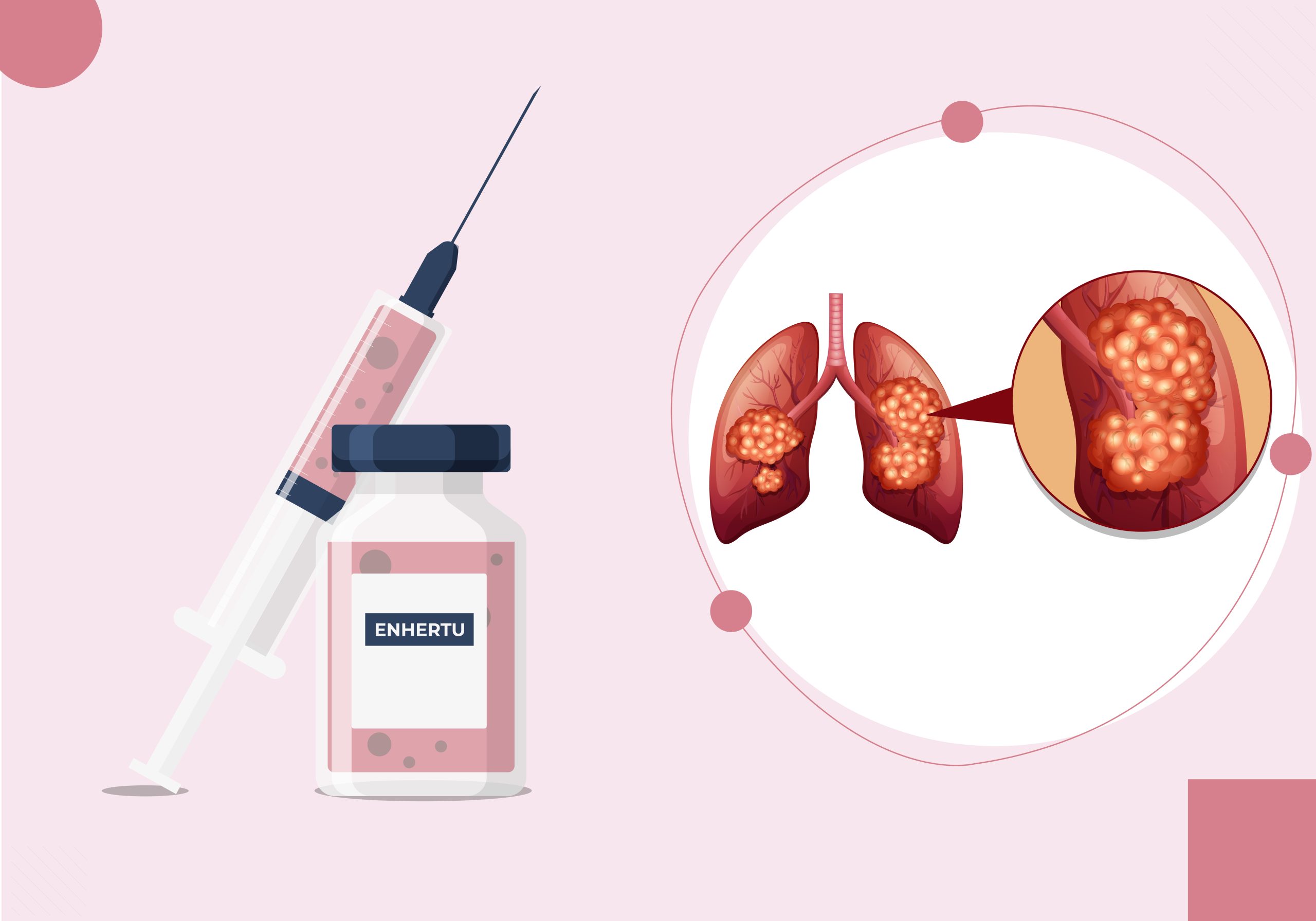
Enhertu (Trastuzumab deruxtecan) is an innovative antibody-drug conjugate (ADC) developed to target HER2 mutations in various cancers, including non-small cell lung cancer (NSCLC). The drug combines three crucial components:
- A topoisomerase I inhibitor (TI), a powerful chemotherapy agent,
- A humanized anti-HER2 IgG1 monoclonal antibody (mAb) that guides the drug to cancer cells, and
- A tetrapeptide-based cleavable linker that ensures the precise delivery of the chemotherapy to the tumor.
This sophisticated drug structure allows Enhertu to deliver its payload with accuracy, limiting damage to healthy cells. Enhertu is manufactured using recombinant DNA (rDNA) technology in CHO (Chinese hamster ovary) cells, with chemical synthesis employed for the TI and linker. Each antibody molecule is linked to approximately 8 molecules of deruxtecan, enhancing its ability to fight cancer effectively.
Enhertu’s Global Impact:
Enhertu has received global recognition for its effectiveness in treating HER2-mutant NSCLC, among other cancers. In August 2022, it was authorized for treating unresectable or metastatic NSCLC in patients with HER2 mutations by the U.S. FDA under its fast-track approval program. This approval was based on an impressive 57.7% overall response rate (ORR) in clinical trials, highlighting the drug’s capacity to shrink tumors in patients who had previously exhausted other treatment options.
This approval has marked a significant milestone in the field of oncology, as Enhertu is now recognized for treating three distinct tumor types: breast cancer, gastric cancer, and NSCLC. Global clinical data continues to support Enhertu’s effectiveness, especially in patients who have progressed on standard therapies.
Enhertu’s Role in NSCLC Treatment:
Lung cancer remains one of the most common and deadliest cancers worldwide. In 2020 alone, there were over 2.2 million new cases of lung cancer and 1.8 million deaths globally, with NSCLC accounting for approximately 85% of these cases. HER2 mutations, while rare, occur in 1-2% of NSCLC patients, creating a critical need for targeted treatments like Enhertu.
A phase-I study of Enhertu in patients with heavily pretreated NSCLC demonstrated a 72.7% ORR, providing a lifeline for patients who have already undergone multiple therapies. With its targeted action, Enhertu offers a promising future for individuals with HER2-mutant NSCLC, significantly improving survival and response rates.
Enhertu’s Broader Applications:
Enhertu has not only revolutionized NSCLC treatment but is also expanding into other cancers. In 2021, the FDA approved Enhertu for treating HER2-positive gastric cancer, making it the first HER2-directed therapy for this cancer type in a decade. The drug is also being studied for other HER2-expressing cancers, including colorectal, bladder, and breast cancers.
The ongoing global clinical trials are expected to provide more robust data on Enhertu’s effectiveness in these additional cancers, positioning the drug as a versatile tool in oncology.
FAQs:
What is Enhertu, and how does it work in NSCLC treatment?
Enhertu (Trastuzumab deruxtecan) is an antibody-drug conjugate that targets HER2 mutations in non-small cell lung cancer (NSCLC). The drug links a monoclonal antibody to a chemotherapy agent, allowing it to deliver treatment directly to cancer cells.
How effective is Enhertu in treating HER2-mutant NSCLC?
In clinical trials, Enhertu demonstrated a 57.7% overall response rate (ORR) in patients with HER2-mutant NSCLC. Additionally, the drug showed a 72.7% ORR in patients who had undergone multiple treatments.
In which countries has Enhertu been approved?
Enhertu has been approved for use in multiple countries, including the United States, Japan, European Union (EU), Australia, and Canada. Its approvals cover various cancers, including HER2-positive breast cancer, gastric cancer, and HER2-mutant non-small cell lung cancer (NSCLC).
Is Enhertu approved for other cancer types?
Yes, Enhertu is approved for treating HER2-positive breast cancer and gastric cancer. It is also under investigation for other cancers like colorectal and bladder cancer.
Can Enhertu be used as a first-line treatment for NSCLC?
Currently, Enhertu is used in patients with unresectable or metastatic NSCLC who have HER2 mutations and have already received prior treatments. It is not yet approved as a first-line therapy.
What are the side effects of Enhertu in NSCLC treatment?
The common side effects of Enhertu include nausea, fatigue, and reduced white blood cell counts. Severe lung problems have also been reported, so patients should be monitored closely by their healthcare providers.
Can I buy Enhertu online from India?
Yes, patients can buy Enhertu online from India through the Named Patient Program. Enhertu can be supplied/exported from countries like India where it is approved. For inquiries and to buy Enhertu in Brazil, Russia, China, Mexico, South Africa, Argentina, Indonesia, Philippines, Vietnam, Saudi Arabia, UAE, Egypt, Turkey, Pakistan, Bangladesh, Sri Lanka, and others, contact Indian Pharma Network (IPN) via Call/WhatsApp: +91 9310090915.
Conclusion:
Enhertu (Trastuzumab deruxtecan) is a more targeted and effective approach to treating cancer, mainly for patients with HER2 mutations in non-small cell lung cancer (NSCLC). As the first HER2-targeted drug approved for NSCLC and with approvals for breast and gastric cancers, Enhertu continues to make strides in oncology, offering new hope to patients worldwide.
As more data emerges from global clinical trials, Enhertu’s use in HER2-expressing cancers is expected to grow, enhancing treatment outcomes for patients who have limited options.
For more information about how to buy Enhertu online, contact Indian Pharma Network (IPN) via Call/WhatsApp: +91 9310090915 to learn about availability through the Named Patient Program.