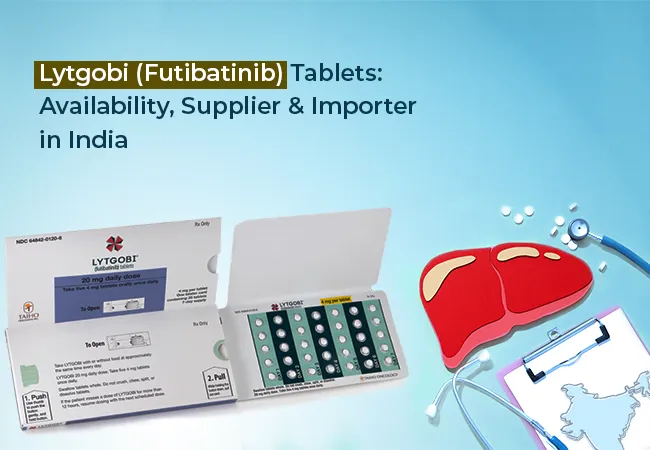
Lytgobi (futibatinib) is a kinase inhibitor. It is used for the treatment of patients with cholangiocarcinoma (bile duct cancer), a rare and aggressive type of cancer with limited treatment options. This therapeutic drug offers new hope for patients with FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma, mainly those whose disease has progressed after prior systemic therapy.
Lytgobi is approved by the U.S. FDA. It is available in selected international markets. Lytgobi in India is not yet commercially available. However, through provisions like Named Patient Import, eligible patients can legally access this medicine under medical prescription.
Lytgobi (Futibatinib): Quick Overview
- Generic Name: Futibatinib
- Brand Name: Lytgobi
- Type: FGFR inhibitor (targeted therapy)
- Form: Oral Tablets (typically 20 mg)
- FDA Status: Approved for FGFR2 fusion-positive intrahepatic cholangiocarcinoma
- Indication: Adults with previously treated, unresectable, locally advanced, or metastatic cholangiocarcinoma with FGFR2 gene fusion or rearrangement
Lytgobi works by selectively blocking the activity of FGFR2 (fibroblast growth factor receptor 2), which is involved in the pathogenesis of some bile duct cancers. Thus, it is another form of precision therapy for patients with that exact mutation.
Availability of Lytgobi in India:
At present, Lytgobi is not commercially approved in India and is not available in retail pharmacies or hospitals. However, patients can still legally access Lytgobi through the Indian Pharma Network (IPN) under a Named Patient Import Program.
This program helps in importing unapproved or hard-to-access medicines for patients in India who have a valid clinician’s prescription and no alternative therapy options exist locally.
Indian Pharma Network (IPN): Trusted Importer & Supplier of Lytgobi in India:
Indian Pharma Network (IPN) is one of the most reliable pharmaceutical importers in India. We specialize in Named Patient Medicine Access. With regulatory expertise and strong connections with global manufacturers, IPN helps patients and doctors access Lytgobi and other unapproved or unavailable medicines safely and efficiently.
Why Choose IPN?
- Authorized Lytgobi Importer in India under CDSCO guidelines
- Trusted by 10,000+ patients across the world
- Regulatory documentation support
- Timely and secure cold-chain logistics (if required)
- Supply to hospitals, doctors, and individual patients
- Transparent pricing and import guidance
How to Import Lytgobi Tablets in India through IPN?
Step-by-Step Process:
- Doctor’s Prescription: The treating clinician must prescribe this therapeutic drug for an eligible patient.
Patient Consent & - Documentation: Submit basic patient details and diagnosis reports confirming FGFR2 fusion.
- Regulatory Approval: IPN receives requisite permissions from CDSCO and DGFT to import the medicine on a named patient basis.
- Sourcing & Shipment: Lytgobi is sourced from authorized vendors in the U.S., EU, or Japan.
- Delivery: IPN promises delivery to the hospital or the patient’s location within 7 to 21 days, depending on availability and logistics.
Who Can Request Lytgobi?
- Patients diagnosed with intrahepatic
- cholangiocarcinoma (iCCA) with FGFR2 fusion
- Oncologists and liver specialists treating rare biliary cancers
- Hospitals and specialty cancer centers seeking Lytgobi for compassionate use or advanced treatment
Alternate Treatment Options for Cholangiocarcinoma:
Lytgobi is especially useful in cases where other systemic therapies have failed. However, other FDA-approved drugs that may be prescribed for cholangiocarcinoma include:
- Pemigatinib (Pemazyre)
- Infigratinib (Truseltiq)
Gemcitabine + - Cisplatin-based Chemotherapy
IPN can help import any of the above medicines on a case-by-case basis under legal frameworks.
Need Help Accessing Lytgobi in India?
Whether you are a patient, caregiver, doctor, or hospital representative, Indian Pharma Network (IPN) is here to help you gain access to advanced cancer treatments like Lytgobi. Contact us to submit your prescription today.
disclaimer: This blog is for informational purposes only. Lytgobi should be taken only under the supervision of a qualified healthcare provider (HCP). All medicines imported through IPN are processed legally under applicable Indian regulatory guidelines.
Is Lytgobi available in India?
No. This therapeutic drug is currently not approved/available in India. However, trusted Indian importers like Indian Pharma Network (IPN) can import this medicine for patients on a Named Patient basis. A valid prescription is required.
Who is the best Lytgobi supplier in India?
Indian Pharma Network (IPN) is a well-known Lytgobi supplier in India. We offer legal, ethical, and timely access to Lytgobi for eligible patients with valid prescriptions.
What is the cost of Lytgobi in India?
Costs are subject to changes because of the country of origin, dosage strength, and supply logistics. For the latest quote, kindly contact IPN directly with a valid prescription.
How long does it take to import Lytgobi in India?
Once the regulatory process is completed, it typically takes 7 to 21 working days to deliver Lytgobi tablets to the patient or hospital.
Is Lytgobi approved by the FDA?
Yes. Lytgobi (futibatinib) is an FDA-approved medicine. It is used for adults with FGFR2-positive intrahepatic cholangiocarcinoma that has progressed after prior treatment.
Can IPN help hospitals or doctors in importing Lytgobi in bulk?
Yes. IPN provides support for bulk or institutional supply requests and can coordinate directly with oncologists and cancer centers to arrange compliant import.