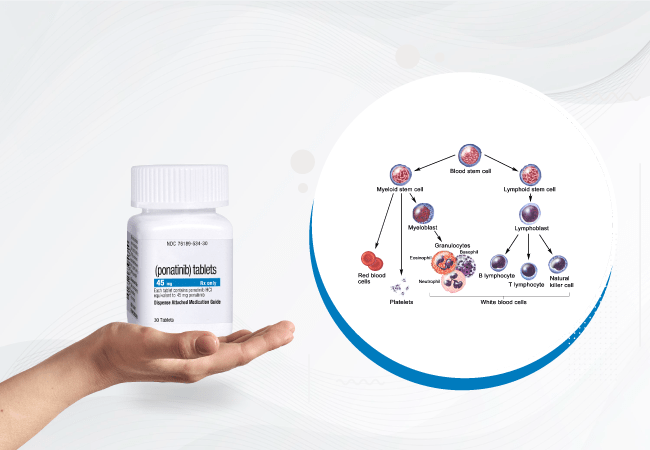
In the realm of Chronic Myeloid Leukemia (CML), Ponatinib has emerged as a groundbreaking agent, particularly in its effectiveness as induction therapy during the chronic phase. Ponatinib, known for its potency against resistant mutations, showcases remarkable efficacy as induction therapy, aiming for swift and deep responses in the chronic phase of CML.
The ability of Ponatinib to target and overcome resistance, including the notorious T315I mutation, positions it as a pivotal player in cases unresponsive to conventional therapies.
Effects of Low-dose Ponatinib in Chronic Phase CML:
According to research presented at the ASH Annual Meeting 2023, Lower-dose ponatinib has shown efficacy as induction in those with chronic phase (CP) chronic myeloid leukemia (CML).
“Ponatinib exerts a sublime antileukemic activity as front-line therapy of chronic phase (CP) chronic myeloid leukemia at 30 mg daily,” stated study presenter Franck E. Nicolini, MD, PhD, of the Centre Léon Bérard in Lyon, France. Dr Nicolini reported that rates of cardiac and vascular toxicity were low, but these events need to be detected as quickly as possible.
Dr Nicolini and their colleagues tested upfront ponatinib in CP chronic myeloid leukemia (CML) in the phase 2 TIPI trial (NCT04070443). The trial was formed to test 6 months of ponatinib induction followed by consolidation with a kinase inhibitor called imatinib, but Dr Nicolini presented findings after ponatinib treatment alone.
Patients enrolled in the trial intended to take ponatinib 30 mg daily for 6 months. Induction is followed by consolidation with imatinib 400 mg daily until treatment-free remission, defined as a molecular response with a 4.5-log reduction (MR4.5) that is sustained for at least 24 months. The trial enrolled and treated 169 patients with chronic phase (CP) chronic myeloid leukemia (CML). Their median age at baseline was 48 (range, 18 to 65) years, and 67 percent were men.
Patients were treated with ponatinib for a median of 174 days, and the median follow-up was 18 months. At 1 month, 93 percent of patients had a complete hematologic response (CHR), and 97 percent had an early molecular response (EMR). At 3 months, 70.5 percent had a complete cytogenetic response (CCyR).
At 6 months, 57 percent of patients reached a major molecular response, 30 percent reached an MR4, 11 percent reached an MR4.5, and 8.3 percent reached an MR5.
The event-free survival rate (SR) at 6 months was 90.53 percent. The events included a couple of deaths – 1 sudden cardiac death and 1 transplant-related death — as well as 14 adverse events (AEs) that caused permanent discontinuation of treatment.
Conclusion:
Ponatinib’s effectiveness as an induction therapy in the chronic phase of chronic myeloid leukemia (CML) marks a significant leap forward in leukemia treatment. As the study continues to unravel its exquisite potential, Ponatinib stands as a beacon of hope, reshaping the landscape of chronic myeloid leukemia (CML) management and inspiring optimism for a brighter future in the fight against this complex disease.
References:
https://ash.confex.com/ash/2023/webprogram/Paper181571.html