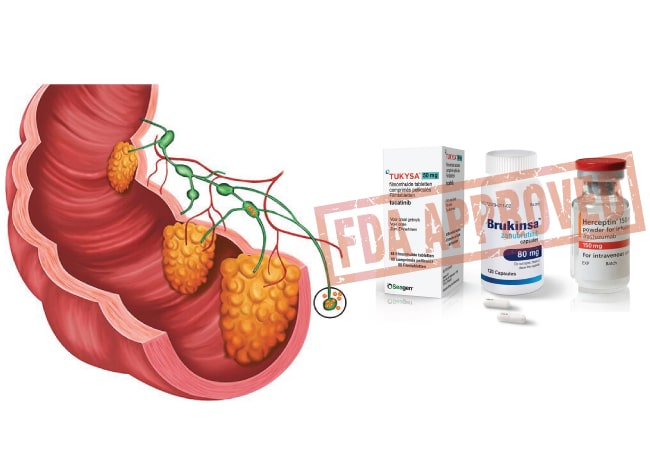
On January 19, 2023 the FDA approved drugs for the treatment of colorectal cancer and small lymphocytic lymphoma.
Tucatinib & Trastuzumab: colorectal cancer
The first approved drug is combination of tucatinib and trastuzumab for RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
Efficacy was evaluated in 84 patients, where patients were asked to have HER2-positive, RAS wild-type, unresectable or metastatic colorectal cancer and prior treatment with fluoropyrimidine, oxaliplatin, irinotecan, and an anti-vascular endothelial growth factor monoclonal antibody. Patients whose tumors were deficient in mismatch repair proteins or were microsatellite instability-high must also have received an anti-programmed cell death protein-1 monoclonal antibody. Professionals removed patients who received anti-HER2 targeting therapy before.
Overall response rate was 38% and median duration of response was 12.4 months.
The most common side effects observed are diarrhea, fatigue, rash, nausea, abdominal pain, infusion related reactions, and pyrexia.
Zanubrutinib: Small lymphocytic lymphoma
The FDA also approved Zanubrutinib (Brukinsa) to treat chronic lymphocytic leukemia or small lymphocytic lymphoma.
In a separate non-randomized cohort of Sequoia, zanubrutinib was evaluated in 110 patients with previously untreated chronic lymphocytic leukemia or small lymphocytic lymphoma with 17p deletion. Overall response rate per independent review committee was 88%.
Efficacy in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma was evaluated in ALPINE. A total of 652 patients were randomized 1:1 to receive either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm and 73% in the ibrutinib arm.Across clinical trials of zanubrutinib, the most common adverse reactions occurring in more than 30% of patients were neutrophil count decreased, upper respiratory tract infection, platelet count decreased, hemorrhage, and musculoskeletal pain.
Reference: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-tukysa-tucatinib-trastuzumab-colorectal-cancer-and-brukinsa#:~:text=On%20January%2019%2C%202023%2C%20the,%2C%20and%20irinotecan%2Dbased%20chemotherapy.